DNA and RNA editing technologies are reshaping biomedical science, enabling precise changes to the genome and transcriptome. While DNA editing allows for permanent genetic modifications, RNA editing enables temporary changes that can be adjusted or reversed, reducing long-term risks. This field has advanced significantly, with groundbreaking therapies addressing genetic disorders and other health conditions. Most recently, Wave Life Sciences, a US biotechnology company, became the first to clinically treat a genetic disorder by directly editing RNA.
RNA Editing: Mechanisms and Potential
Overview of RNA Editing
RNA editing involves modifying messenger RNA (mRNA) nucleotides after DNA transcription but before protein synthesis. The mRNA molecule is structured with exons (coding sections) and introns (non-coding sections). During RNA editing, specific changes are made to exons to potentially alter the resulting proteins, while introns are typically removed.
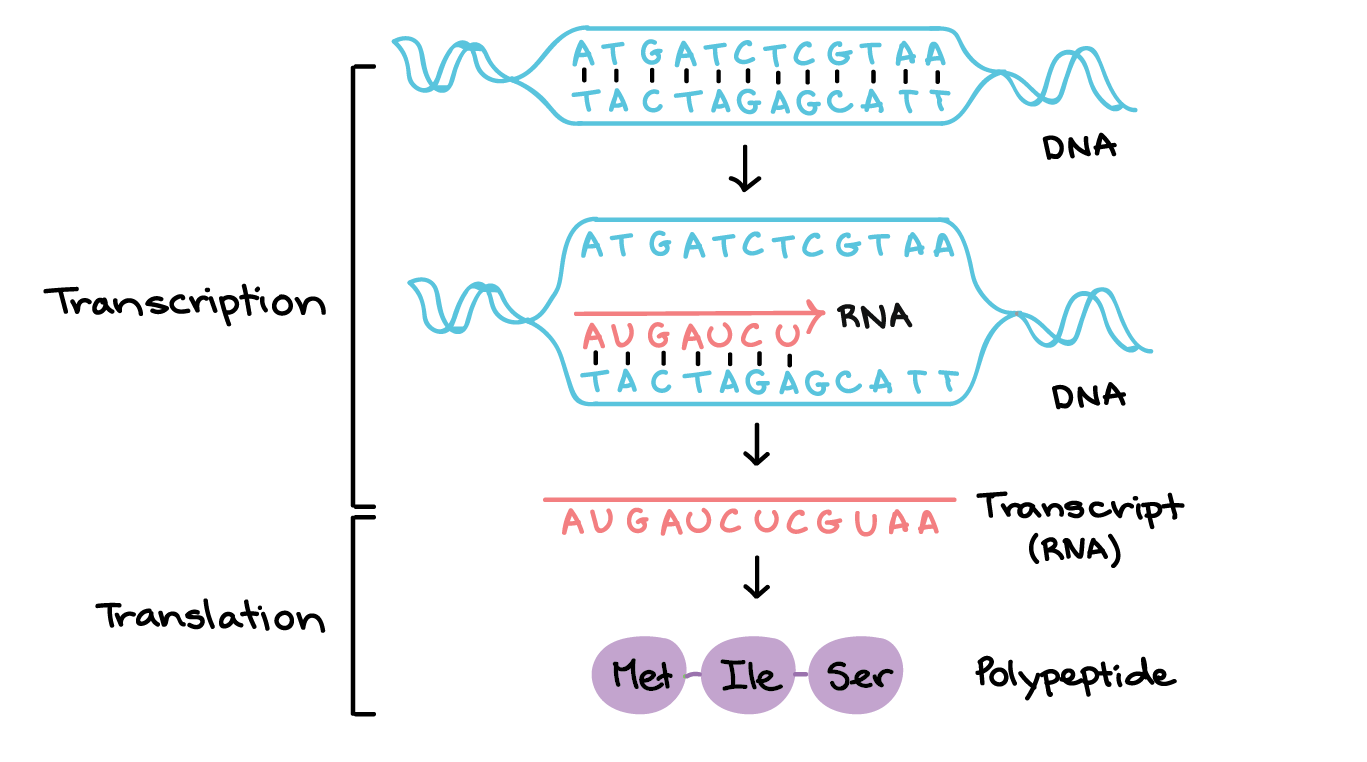
Key forms of RNA modifications include:
- Addition: A nucleotide is inserted into the RNA strand.
- Deletion: A nucleotide is removed from the RNA strand.
- Substitution: One nucleotide is replaced by another.
Mechanism of RNA Editing
RNA editing typically involves adenosine deaminase acting on RNA (ADAR) enzymes, which target and modify adenosine bases within RNA. ADAR operates in tandem with a guide RNA (gRNA), directing it to specific mRNA regions. This targeted approach allows for selective and precise modification of RNA, enabling the correction of potentially harmful genetic instructions without changing the DNA itself.
Clinical Applications of RNA Editing
Wave Life Sciences used RNA editing to treat alpha-1 antitrypsin deficiency (AATD), a genetic disorder, through their WVE-006 therapy. RNA editing shows promise for treating various conditions, including Huntington’s disease, Parkinson’s disease, Duchenne muscular dystrophy, neurological disorders, obesity, and heart disease.
Challenges in RNA Editing
RNA editing poses some challenges, primarily due to its temporary nature. It requires repeated treatments for lasting effects, unlike DNA editing. Additionally, current delivery systems, such as lipid nanoparticles and adeno-associated virus (AAV) vectors, face limitations with large molecule transport, creating hurdles in clinical applications.
Fundamentals of RNA
Definition and Structure of RNA
RNA is a single-stranded nucleic acid present in all living cells, serving as the intermediary in gene expression from DNA to protein. It is composed of a backbone of alternating phosphate groups and ribose sugars and includes the nitrogenous bases adenine (A), uracil (U), cytosine (C), and guanine (G).
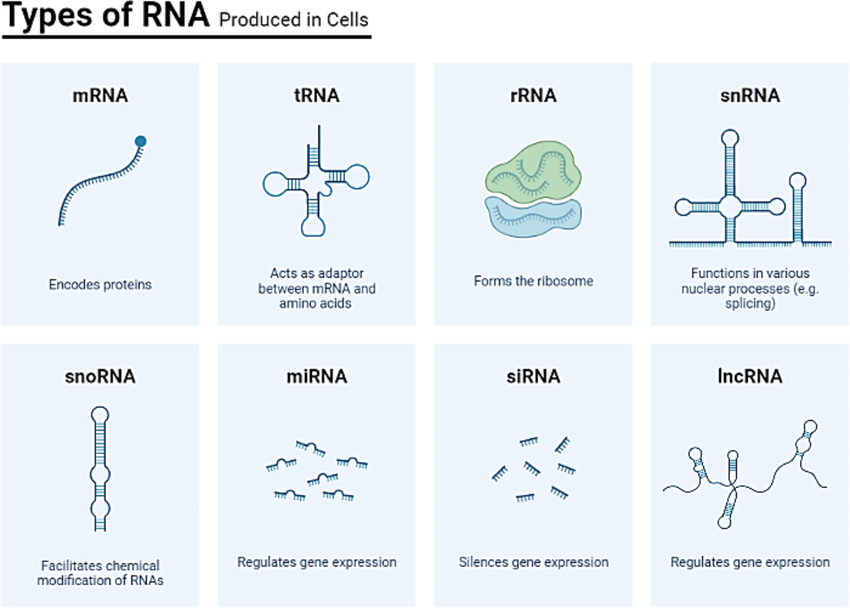
Types of RNA
- Messenger RNA (mRNA): Carries genetic information from DNA to ribosomes for protein synthesis.
- Ribosomal RNA (rRNA): Forms the core structure of ribosomes and catalyzes protein assembly.
- Transfer RNA (tRNA): Transfers amino acids to the ribosome during protein synthesis.
- Regulatory RNAs: Involved in gene expression regulation and play roles in silencing and enhancing specific genes.
Role of RNA in Viruses
Some viruses, such as coronaviruses and influenza, use RNA as their genetic material, making RNA editing a potential tool for antiviral therapies. In this context, RNA’s function as genetic material is a point of vulnerability that could be exploited for therapeutic interventions.
DNA Editing vs. RNA Editing
Permanence and Temporariness
- DNA Editing: Produces permanent genetic changes, which can offer lifelong solutions for genetic disorders but may result in irreversible errors.
- RNA Editing: Alters RNA temporarily, allowing for flexible, reversible modifications that minimize long-term risks. This flexibility enables discontinuation if adverse effects arise.
Immune Response Considerations
- DNA Editing: Often involves tools like CRISPR-Cas9, derived from bacterial systems, which can provoke immune responses due to foreign protein exposure.
- RNA Editing: Utilizes ADAR enzymes naturally present in human cells, significantly reducing the risk of immune or allergic reactions. This approach is better suited for individuals with immune sensitivities and allows for repeated treatments.
Structure and Function of DNA
Basic Composition
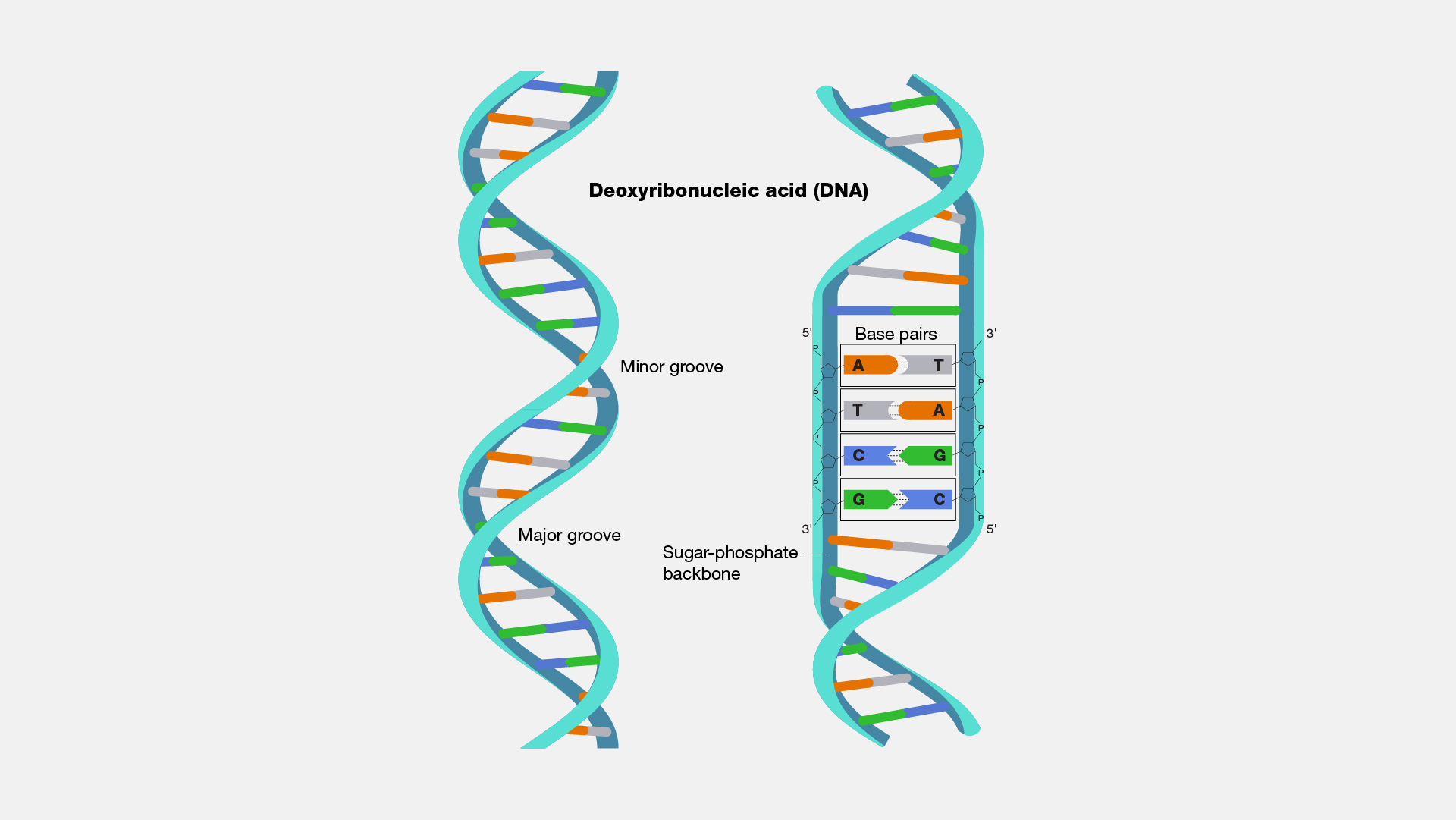
DNA, the double-stranded molecule central to genetic storage, consists of nucleotides, each containing:
- A phosphate group
- A deoxyribose sugar
- A nitrogenous base (adenine [A], thymine [T], cytosine [C], or guanine [G])
DNA’s structure is often visualized as a double helix, where adenine pairs with thymine (A-T) and cytosine pairs with guanine (C-G) through hydrogen bonds.
Double Helix Structure
The double helix structure of DNA, first proposed by Watson and Crick in 1953, illustrates the antiparallel orientation of the two strands. This configuration is essential for replication and transcription, ensuring the accurate transfer of genetic information. Each helix turn contains 10 base pairs with a pitch of approximately 3.4 nanometers.
Chromosomal Organization in Eukaryotes and Prokaryotes
In eukaryotic cells, DNA organizes into chromosomes located in the nucleus. Each chromosome includes a long DNA molecule packed with histone proteins for efficient storage. In contrast, prokaryotes have circular DNA that resides in the cytoplasm without histones.
Function of DNA in Genetic Information Storage
DNA functions as the repository of genetic instructions necessary for the growth, development, and reproduction of organisms. It encodes protein-building information through gene expression, a process involving transcription (DNA to RNA conversion) and translation (RNA to protein synthesis).
DNA Replication
The replication process is semiconservative, where each DNA strand serves as a template for the formation of a new strand, ensuring that each daughter cell receives an exact DNA copy. Key steps include:
- Initiation: DNA unwinds at origins of replication.
- Elongation: DNA polymerase adds complementary nucleotides.
- Termination: Replication completes with a fully duplicated DNA molecule.
Gene Expression
Gene expression, involving transcription and translation, enables DNA instructions to become proteins:
- Transcription: RNA polymerase transcribes specific DNA segments into RNA.
- Translation: Ribosomes use mRNA instructions to assemble proteins by linking amino acids.
RNA Functionality in Protein Synthesis
RNA is crucial in translating genetic information into functional proteins. This occurs via:
- Transcription: mRNA synthesis from DNA.
- Translation: Ribosomes read mRNA, and tRNA delivers amino acids to build a protein chain.
RNA’s Role in Gene Regulation
Certain RNA types, such as small interfering RNAs (siRNAs) and microRNAs (miRNAs), participate in post-transcriptional regulation, affecting mRNA stability and gene expression patterns.
Editing Techniques in DNA and RNA
DNA Editing Methods
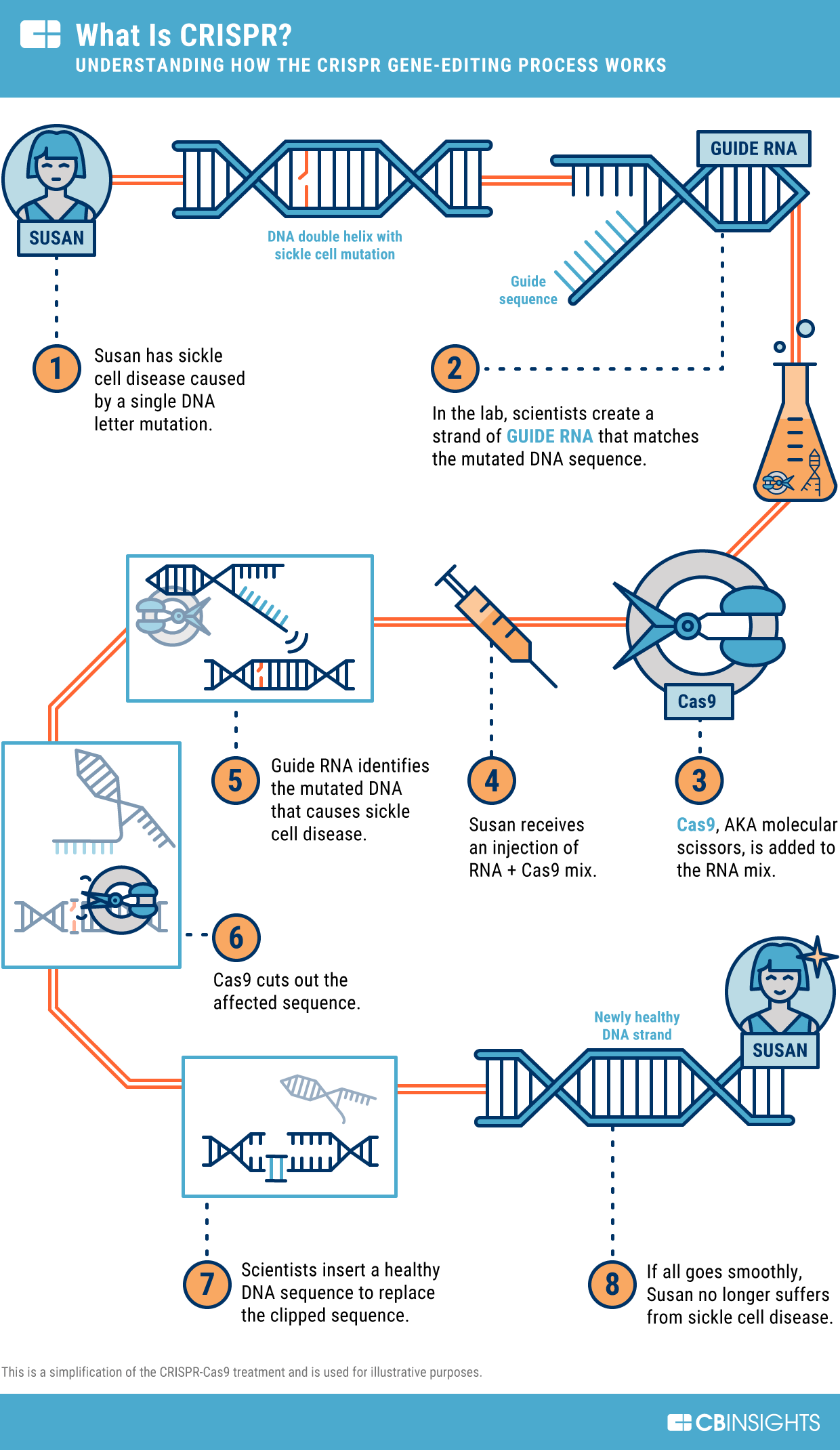
- CRISPR-Cas9 Technology: Revolutionizing DNA editing, CRISPR-Cas9 enables precise genetic modifications.
- Mechanism: A guide RNA directs the Cas9 enzyme to a specific genome site, where it introduces a double-strand break. Natural repair mechanisms then alter the DNA sequence as desired.
- Homologous Recombination: This method uses homologous DNA sequences to integrate specific genetic changes. It requires a donor template with the desired alterations, allowing for accurate sequence replacements.
- Base Editing: Unlike CRISPR, base editing converts specific nucleotide pairs without double-strand breaks. It uses engineered enzymes to transform A-T to G-C pairs or vice versa with high precision.
RNA Editing Techniques
- A-to-I Editing: Converts adenosine (A) to inosine (I) in mRNA, altering protein coding and mRNA splicing patterns. This technique is versatile, allowing specific post-transcriptional modifications.
- CRISPR-based RNA Editing: Adapting CRISPR for RNA allows researchers to target mRNA for degradation or adjustment. Guide RNAs direct the Cas proteins to mRNA, enabling selective transcript modifications.
- Alternative Splicing: By selecting different combinations of exons, cells can produce multiple protein forms from a single gene. This natural editing method significantly increases protein diversity without modifying DNA sequences.
The Future of Genetic and Transcriptomic Editing
As RNA and DNA editing technologies advance, the potential for treating diseases once considered incurable grows. While DNA editing offers lasting solutions, RNA editing provides a temporary, flexible approach suited to conditions where gene expression needs precise control. Both fields have transformative potential, but ongoing research is essential to refine these techniques, address ethical concerns, and improve delivery mechanisms for clinical applications.
As these technologies develop, they promise to redefine possibilities for treating genetic disorders, enhancing personalized medicine, and exploring the complex interplay between genomics and transcriptomics.